
#CHEMICAL EQUATION FOR LATTICE ENERGY OF NACL HOW TO#
In this video I will discuss lattice energy and how to use the Born-Haber cycle to calculate lattice energy from known enthalpies of certain reactions. Where all the numbers you plug in are positive.Connect to WiFi to avoid cellular charges for video streaming. #color(blue)(DeltaH_"lattice" -= -|DeltaH_"lattice"|)# Solving for #DeltaH_"lattice"# usually gives a positive answer, so we take the negative of the answer by convention to get: Take the step as being upwards to generate a complete cycle, for which #DeltaH_"cycle" = 0# (since #H_f = H_i# for a complete cycle). Put that together by adding all the given energies, and you'll have the following diagram: #"These cancel out completely upon adding, proving"# Then, the following reactions would be involved to transform each reactant for the formation of #bb("1 mol")# of #"NaCl"(s)# (the units therefore become #ulul"kJ"#!): #1/2"Cl"_2(g) stackrel(1/2xx"Bond-Breaking")overbrace(->) "Cl"(g) stackrel("1st Electron Affinity")overbrace(->) "Cl"^(-)(g)#įor simplicity in our Born-Haber cycle, let up use positive numbers and down use negative numbers. Our goal is to fill in the missing steps by transforming each element one step at a time (phases, charges, and chemical bonds): This is allowed since enthalpy is a state function, which does not depend on the path.Īs it turns out, the lattice energy is difficult to acquire in real life, so it is often indirectly obtained from calculations not unlike the ones we're doing. This would be what we call the Born-Haber thermodynamic cycle. These share the same product, so let's say we wanted to form the following cycle: Since forming the lattice stabilizes the components (we went from two gases to one solid, creating order), decreasing their overall energy. Note that there exists a negatively-signed lattice energy for the following reaction: Chlorine is a diatomic gas at room temperature and pressure.
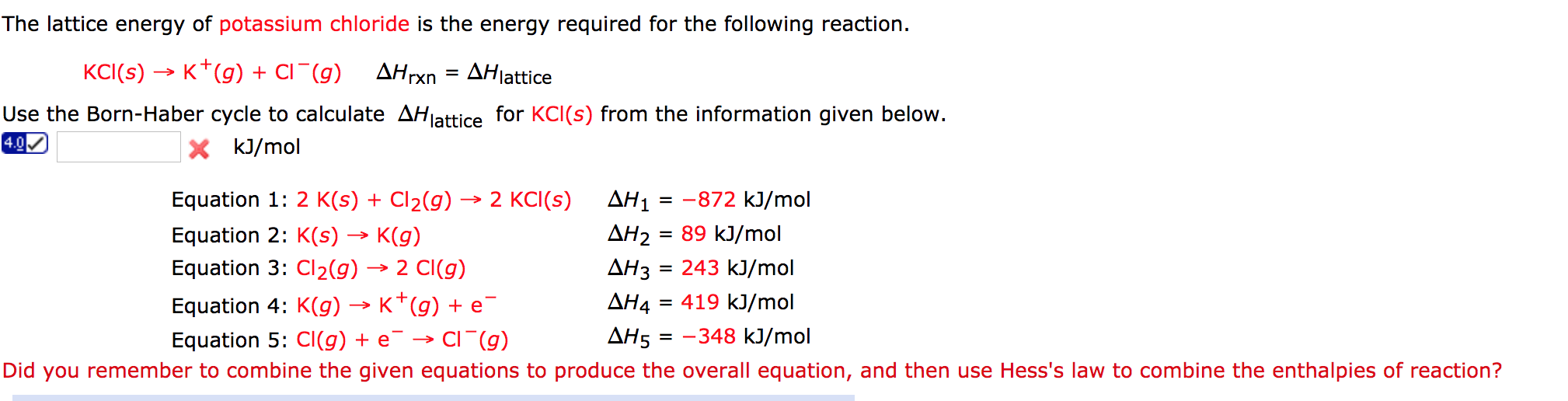
Sodium is a metal at room temperature and pressure.(You should check your textbook to see whether it is defined as #"1 atm"# or #"1 bar"#.)Įlemental state simply means the natural state you find them at typical life conditions. Where all the numbers you plug in to the righthand side are positive.įormation reactions generate #"1 mol"# of the product, such as #"NaCl"(s)#, from the individual elements ( #"Na"#, #"Cl"#) in their elemental state at "C"# and standard pressure. #DeltaH_"lattice" -= -|DeltaH_"lattice"|# Do you mean the energy involved in the process to form #"NaCl"(s)# in its formation reaction? Well, to form the lattice, we have to get to the ions in their gaseous state and then allow sodium to transfer its electron over to chlorine.


 0 kommentar(er)
0 kommentar(er)
